EcoMark laser engraving and laser marking
in medical technology
The medical sector is characterised by a very high degree of regulatory requirements for the quality and safety of products. Therefore, special challenges also arise when marking and labelling medical devices and products. With EcoMark laser machines, you are optimally equipped for all requirements.
ECOMARK LASER MARKING:
PERFECTION IN A REGULATED ENVIRONMENT
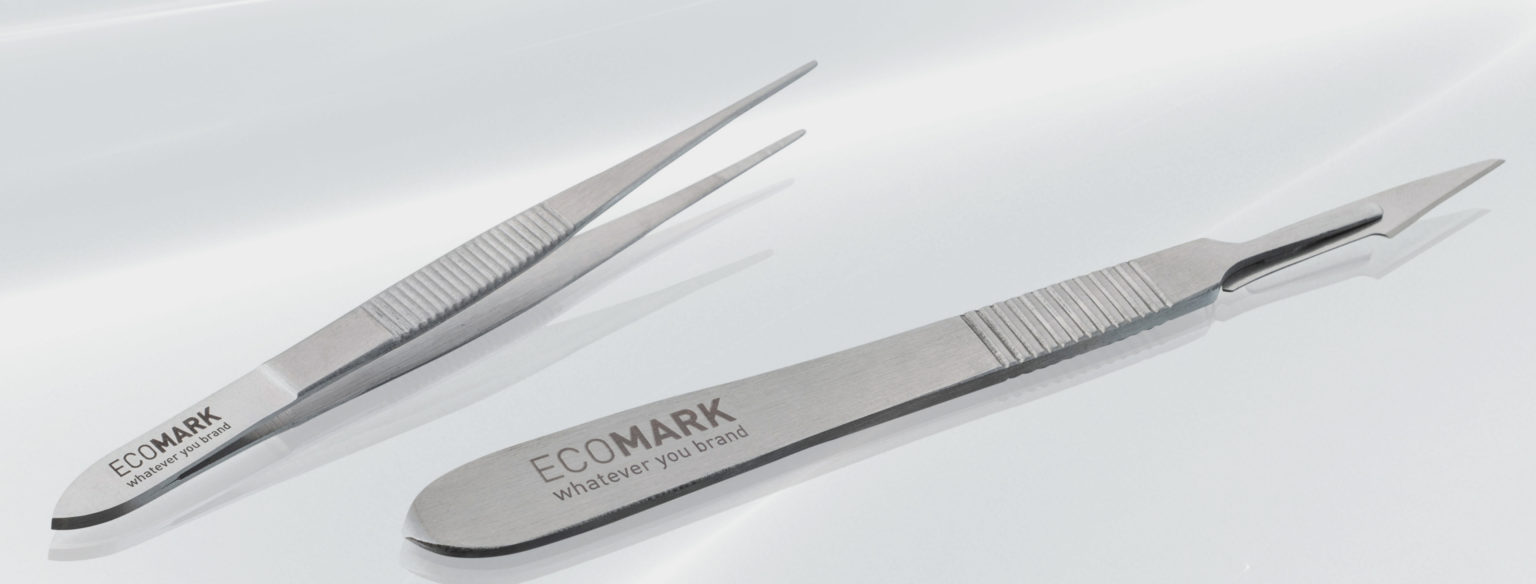
For medical devices and products, such as surgical instruments, implants, cannulas, injection needles, laboratory containers, etc., highly precise, sometimes micro-fine laser markings on plastics, metal and glass are required that are indestructible under all circumstances. Especially with invasive surgical products, there must be no risk of the marking becoming detached, causing injuries or germs settling. Therefore, absolutely reliable top quality in laser marking is required here.
Labelling in medical technology is used, among other things, for safe use, such as measuring scales. Above all, however, the focus is on counterfeit protection and complete traceability along the entire supply chain, especially in the case of implants.
SPECIFIC CHALLENGES
TO LASER MARKING IN MEDICAL TECHNOLOGY
Maximum resistance: Medical technology cutlery and products are cleaned frequently and intensively during use. This means that the laser marking must be able to withstand extreme chemical and mechanical stress as well as high temperatures.
Unique traceability - UDI: Medical devices must be marked in such a way that all relevant information such as origin, manufacturer, production batch, date of manufacture etc. can be permanently determined. This is what the keyword UDI - Unique Device Identification stands for. For certain products that are implanted or that are used several times, marking directly on the object is mandatory. Whether it is glass, medical stainless steel or special plastics - the labelling must remain clearly legible throughout the entire product life cycle.
High quality and safety requirements - Equipment Qualification (EQ): No other production sector is as regulated as the medical sector, and rightly so. The requirements also include manufacturing equipment, including laser marking machines used in medical production. According to the FDA recommendation, equipment qualification includes requirements for Design Qualification (DQ), Installation Qualification (IQ), Operation Qualification (OQ) and Performance or Process Qualification (PQ).
THE ECOMARK INDUSTRY PARTNERSHIP:
CONSULTING, DEVELOPMENT, SOLUTIONS
EcoMark solutions ensure the reliable quality and cutting-edge technology that reliably enables all these requirements. In addition, our machines are equipped with automatic quality control so that defective parts are ejected from further processing. Furthermore, we offer the software interfaces for the database connection and documentation required for UDI-compliant marking.
Talk to us - we will provide you with comprehensive advice and work with you to implement your customised solution for laser marking that meets your needs and the applicable requirements. Both through perfect, durable laser results as well as through efficient, reliable and economical processes with perfect connection to your production environment.
- TALK TO US ABOUT YOUR IDENTIFICATION PROCESSES: We will advise you comprehensively about your advantages and possibilities. And if we don't have a ready-made solution yet, we'll find it together with you!
LIMITLESS AREAS OF APPLICATION
Information for traceability, quality control or compliance with legal requirements, protection against brand piracy and plagiarism, classic branding with brand logos, infographics or machine-readable coding for automated production control: the spectrum of possible and useful applications of laser marking is endless.

TAILOR-MADE: INDUSTRY SOLUTIONS FROM ECOMARK
Well-founded application know-how is the basis for optimal solutions that inspire our customers. As an experienced partner for industrial laser marking, we know your industry and can advise and support you individually in your specific needs.

NOBLE BRAND DESIGN
Precise laser engraving of high-quality aluminium profiles with different lengths and surface coatings.


Traceable coding
The aim was to apply a DMC code to a die-cast aluminium gearbox housing by laser marking.




